- Surging Stock Price: ClearPoint Neuro’s stock has soared in 2025, climbing from single digits into the $20+ range. As of early November 2025, CLPT trades around the mid-$20s per share after hitting a 52-week high near $30marketbeat.commarketbeat.com. Heavy trading volume (nearly 1 million shares on peak days) reflects heightened investor intereststockanalysis.com.
- Business Overview: ClearPoint Neuro is a medical device innovator specializing in MRI-guided neurosurgical platforms. Its ClearPoint system enables minimally invasive brain surgeries – guiding deep brain stimulation (DBS) implants, biopsies, laser ablation catheters, and even direct drug infusions into the brainnasdaq.com. The company has pivoted to become a “cell and gene therapy-enabling” platform for biotech partners, positioning itself at the forefront of next-generation neurological treatments.
- Recent Catalysts: In late 2025 ClearPoint reported multiple breakthroughs. It unveiled a new robotic neuro-navigation system to automate cranial procedures and support high-volume cell/gene therapy deliverystocktitan.net. Its ClearPoint Prism laser system won expanded FDA clearance for use with widely-used 1.5T MRI scanners, opening a larger market (1.5T machines are ~60% of MRI usage)nasdaq.com. ClearPoint’s tools were also used in the first-ever commercial gene therapy delivered to the brain (for AADC deficiency), marking a milestone in neurosurgeryir.clearpointneuro.comir.clearpointneuro.com. Additionally, the company secured regulatory clearances across 34 countries for its neuro delivery devicestockanalysis.com, bolstering its international reach.
- Financial Momentum: Revenues are growing strongly (31% growth in 2024, with 2025 guidance of $36–$41 millionnasdaq.com). Latest quarterly sales hit record levels – Q2 2025 revenue was $9.2M (+17% year-on-year)accessnewswire.com – though the firm remains unprofitable (TTM net loss ~$22M) as it reinvests in growth. Analysts expect Q3 2025 revenue around $9.6M with a –$0.20 EPS lossmarketbeat.com. Balance sheet: ClearPoint bolstered its cash reserves via a $33.5M financing from Oberland Capital in 2025, part of up to $110M available to fund expansionir.clearpointneuro.comir.clearpointneuro.com.
- Analyst Sentiment: Wall Street is bullish on CLPT. All covering analysts rate it a Buy, with price targets ~$28–$30 (averaging ~$29)directorstalkinterviews.com. That implies ~20% upside from recent prices. Experts highlight ClearPoint’s unique position as the “pick-and-shovel” provider for emerging brain therapies – a strategy that could “position [ClearPoint] at the forefront of advancements in neurosurgery and brain therapeutics”directorstalkinterviews.com. However, they also caution that profitability remains a hurdle and execution is key.
- Risks to Watch: ClearPoint’s fortunes are increasingly tied to partner successes. A stark reminder came on Nov 3, 2025 when partner uniQure announced the FDA deemed its Huntington’s gene therapy data “inadequate” for approval – a shocking reversal that sent uniQure’s stock down ~68%kfgo.comkfgo.com. ClearPoint’s stock also wavered on the news. The company faces competition from larger medtech players (Medtronic, Brainlab, Renishaw) who could develop similar MRI-guided neurosurgery toolsmvcinvesting.substack.com. Additionally, ClearPoint is not yet profitable, carries significant accumulated losses, and may need to continue investing heavily to support its 60+ ongoing partner trials.
Stock Performance & Trading Trends (2025)
ClearPoint Neuro’s stock has delivered dramatic gains in 2025, transforming from a little-known small-cap into a high-flyer. The share price rallied over 300% from 2024 lows, driven by positive news in both the company’s own product line and its partners’ clinical trial successes. Notably, CLPT climbed from roughly $5.50 in May 2024 to over $19 by February 2025nasdaq.com. After a mid-year breather, momentum returned in Q3/Q4 2025: the stock surged nearly 40% in one day on September 24, 2025 after announcing an FDA clearance (for its Prism laser) and bullish revenue guidancenasdaq.comnasdaq.com. By late October, ClearPoint hit new highs around $30, before settling in the mid-$20s as of early Novembermarketbeat.commarketbeat.com.
This volatility reflects increasing trading activity and investor attention. Daily volumes have spiked during news events – for example, nearly 916,000 shares traded around late October when the stock jumped, far above normal levelsstockanalysis.com. The stock’s 50-day average price (~$18) is now well above its 200-day average (~$14)directorstalkinterviews.com, confirming a strong uptrend through much of 2025. However, recent swings also underscore risk: on October 30, 2025, CLPT slid about 10% in one day to ~$24marketbeat.com, potentially as investors took profits ahead of earnings or reacted to broader market turbulence. Overall, volatility is high – the 52-week range spans from $9.76 to $30.10marketbeat.com – but the trajectory remains positive year-to-date.
Trading sentiment has been buoyed by ClearPoint’s emerging role in the hot field of gene therapy delivery. Whenever a partner reports positive clinical results, CLPT tends to rally in sympathy. For instance, in September 2025 uniQure announced encouraging Phase I/II data for a Huntington’s disease gene therapy (delivered using ClearPoint’s system), causing ClearPoint’s stock to leap alongside uniQure’s ~200% surgekfgo.com. Conversely, negative surprises can spark pullbacks. This dynamic was evident in early November when the FDA’s unexpected pushback on that same Huntington’s therapy sent shockwaves – ClearPoint’s shares recoiled from recent highs as investors recalibrated the timelines and probabilities of its partners’ programs.
In terms of valuation, the recent ~$25 stock price gives ClearPoint a market capitalization around $650–$700 millionmarketbeat.commarketbeat.com. This reflects a rich multiple on current revenues (20x+ annual sales), suggesting the market is pricing in significant future growth from the company’s technology being widely adopted. The stock’s beta ~1.2 indicates slightly higher volatility than the overall marketmarketbeat.com. No dividend is offered – ClearPoint is squarely in growth mode, reinvesting all capital. Going forward, trading catalysts will include quarterly earnings results, FDA approvals for partner therapies, and any major partnerships or device approvals. Investors should be prepared for large swings around such events.
Company Background & Business Overview
ClearPoint Neuro, Inc. is a medical device company at the intersection of neurosurgery and biotechnology. Founded in 1998 (originally as MRI Interventions, Inc.), ClearPoint has spent decades developing technology to navigate and treat the brain under MRI guidance. The company rebranded to “ClearPoint Neuro” in 2020 to reflect its broadened mission of enabling cutting-edge neurotherapies. Headquartered in Solana Beach, California, ClearPoint now has about 115 employees and operates globallystockanalysis.comir.clearpointneuro.com.
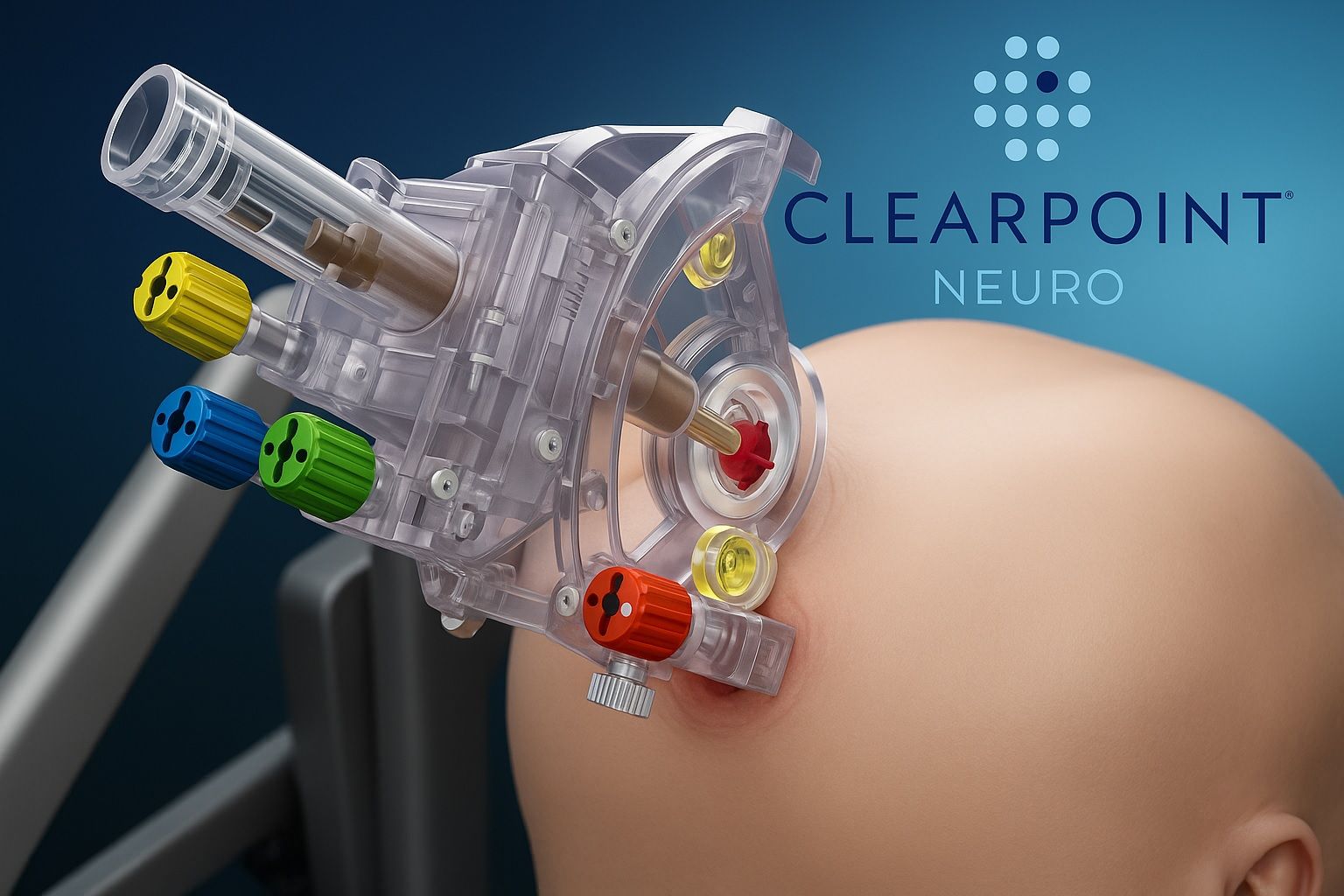
Core Business: ClearPoint’s flagship product is the ClearPoint Neuro Navigation System, a suite of hardware and software that allows neurosurgeons to perform minimally invasive procedures inside the MRI suite. The system provides real-time, MRI-guided navigation for inserting instruments or delivering therapies to precise brain targets. Originally, ClearPoint’s focus was on functional neurosurgery procedures like:
- Deep Brain Stimulation (DBS): guiding electrode implantation for Parkinson’s, epilepsy, and other disorders. Using ClearPoint, these surgeries can be done with the patient under general anesthesia (avoiding awake brain surgery) while still ensuring accurate placementcstproxy.comcstproxy.com.
- Laser Ablation (LITT): placing laser catheters into brain tumors or epilepsy foci. ClearPoint partnered with Clinical Laserthermia Systems (CLS) to develop the Prism Neuro Laser Therapy System, which gained FDA clearance in 2022 and targets conditions like glioblastomas and drug-resistant epilepsycstproxy.comcstproxy.com.
- Brain Biopsies: navigating needles to hard-to-reach brain lesions for tissue sampling, improving safety and yield for deep or delicate tumorscstproxy.com.
These MRI-guided device applications address significant markets (tens of thousands of potential procedures per year in the U.S. for DBS, laser ablation, etc.cstproxy.comcstproxy.com). ClearPoint generates revenue by selling the capital equipment (the software, workstation, and disposable surgical components) and servicing these systems at hospital sites.
Evolution to Therapy Delivery: In the last few years, ClearPoint has strategically expanded into a second segment: enabling direct drug, gene, and cell therapy delivery to the brain. The company recognized that many next-generation neurological treatments – from gene therapies for rare pediatric diseases to novel oncology drugs – require bypassing the blood-brain barrier and infusing the therapy precisely into brain tissuecstproxy.comcstproxy.com. ClearPoint’s solution is the SmartFlow® cannula and its navigation platform, which together allow pharmaceutical partners to safely inject therapeutics into specific brain regions under MRI guidance.
This move has opened a new growth avenue: partnering with biotech and pharma companies in their clinical trials. ClearPoint provides the specialized devices, software, and support for these experimental neurosurgical infusions. As of 2025, ClearPoint reports working with 60+ biopharma partners worldwide on preclinical and clinical programsir.clearpointneuro.com. The therapies span rare genetic disorders (like AADC deficiency, Friedreich’s ataxia), more common neurological diseases (Parkinson’s, epilepsy, Huntington’s), and even brain cancerscstproxy.comcstproxy.com. Essentially, ClearPoint’s technology is becoming the de facto delivery platform for a pipeline of cutting-edge neurology drugs.
Leadership: ClearPoint’s management blends medtech and biotech expertise. Joseph “Joe” Burnett, President and CEO, has led the company since 2017 and is a vocal champion of its vision to “transform patient therapy” via neuro-navigationir.clearpointneuro.com. CFO Danilo D’Alessandro orchestrated recent financings and emphasizes aligning the company with partners’ needsir.clearpointneuro.com. In September 2025, ClearPoint appointed neurosurgeon Dr. Paul Larson as Chief Medical Officer, underscoring its close ties with the surgical communityir.clearpointneuro.com. The board of directors gained seasoned industry veterans, including (as of late 2025) José Bayardo – a finance executive added to help guide the company’s growth (noted in an earnings announcement). This mix of technical, clinical, and financial leadership reflects ClearPoint’s dual focus as a medical device innovator and an enabler for pharma.
Target Market: ClearPoint’s customers and end-users fall into two groups:
- Hospitals and Neurosurgeons – institutions that perform brain surgeries and want state-of-the-art MRI-guided capabilities. ClearPoint’s installed base has grown to dozens of major neurosurgical centers across North America, Europe, and beyondir.clearpointneuro.com. These sites use the ClearPoint system for DBS, biopsies, laser ablations and, increasingly, for clinical trial infusions. As more therapies gain approval, every hospital treating those patients may need ClearPoint’s system, a point the company emphasizes: if their device is listed in a therapy’s label, any hospital offering that treatment “must have ClearPoint” technology on handir.clearpointneuro.com.
- Biotech/Pharma Partners – companies developing central nervous system (CNS) therapies who require a delivery method. ClearPoint’s partners range from large pharmaceutical firms (e.g. UCB Biopharma, which collaborates on certain neuro programsdirectorstalkinterviews.com) to startups and academic spin-offs. Notable partners cited include Koninklijke Philips N.V. (for imaging integration), Aspen Neuroscience (autologous cell therapy for Parkinson’s), PTC Therapeutics (gene therapy for AADC deficiency), and many others. ClearPoint earns revenue from these partners via the sale of disposable cannulas, specialized services, and consulting on trial design. This “picks and shovels” strategy means ClearPoint benefits from a broad swath of drug programs without betting on any single therapy – though success of those drugs would dramatically expand ClearPoint’s device demand.
In summary, ClearPoint Neuro has evolved from a niche MRI-guided surgery player into a platform company straddling medtech and biotech. Its market now encompasses both the traditional neurosurgery device segment and the burgeoning neuro-therapeutics delivery space. This unique positioning – as one analyst put it, “a compelling mix of innovation, growth potential, and the chance for significant returns despite current financial challenges”directorstalkinterviews.com – underpins the recent excitement around CLPT stock.
Recent News and Developments (Late 2025)
The closing months of 2025 have been eventful for ClearPoint Neuro, with a flurry of news on product advances, partnerships, and financial results. Here are the key recent developments up to November 3, 2025:
- Q3 2025 Earnings on Deck: ClearPoint is slated to report third quarter 2025 results on November 6, 2025. According to consensus, analysts expect ~$9.6 million in revenue and an EPS of –$0.20 for the quartermarketbeat.com. This would represent continued double-digit growth from the $8.2M revenue in Q3 2024 (estimated) but also ongoing losses as the company invests heavily in R&D and commercialization. In the previous quarter (Q2 2025), ClearPoint delivered record quarterly revenue of $9.2M (+17% YoY)accessnewswire.com, indicating solid momentum. Investors will be watching the Q3 report for updates on full-year guidance (currently $36–$41M revenue for 2025, reaffirmed by managementnasdaq.com) and any changes given recent events. Notably, ClearPoint’s CEO Joe Burnett has described the company’s strategy as “Fast. Forward.” – prioritizing rapid growth in both device placements and partner supportaccessnewswire.comaccessnewswire.com. Any commentary on 2026 outlook, cash burn, or new partnerships during the Q3 conference call will be closely parsed.
- First Gene Therapy to the Brain (AADC Deficiency): In August 2025, ClearPoint announced a watershed moment: its SmartFlow cannula was used in the first-ever commercial gene therapy delivered directly into the brainir.clearpointneuro.comir.clearpointneuro.com. The therapy, called KEBILIDI™ (eladocagene exuparvovec-tneq), is a gene replacement treatment for a rare pediatric disorder (AADC deficiency) that was approved in the U.S. earlier in 2025. ClearPoint’s device is the only FDA-authorized tool for delivering this therapy into the putamen region of the brainir.clearpointneuro.com. In July 2025, neurosurgeons in Texas and Boston successfully performed the first patient infusions of KEBILIDI using ClearPoint’s systemir.clearpointneuro.com. Why it matters: This is a proof of concept for ClearPoint’s role in commercial-stage therapeutics, not just clinical trials. CEO Joe Burnett celebrated it as a “pivotal milestone” – “the first commercial gene therapy treatments in the US ever delivered to the brain”ir.clearpointneuro.comir.clearpointneuro.com. Leading pediatric neurosurgeons involved commented that “direct delivery of cell and gene therapy into the brain represents…a transformation in care…We can now treat the disease, not just the symptoms”ir.clearpointneuro.com. For hospitals and investors, this milestone broadcasts that the age of gene therapy in neurosurgery has arrived, with ClearPoint’s platform enabling it. It also likely opens a modest revenue stream as KEBILIDI treatments roll out (each patient treated will use disposables and possibly generate service revenue for ClearPoint). More importantly, it signals what future approved therapies could bring. Management noted that if their device continues to be included on drug labels as part of a combination product, “it is hard to imagine a situation where ClearPoint is not an essential part of any hospital that wants to participate…in neuro cell and gene therapy”ir.clearpointneuro.com.
- Partner Pipeline Progress: ClearPoint’s August 4, 2025 press release detailed numerous biologics partnership milestones:
- Several partner programs achieved regulatory fast-track or expedited review status at the FDAir.clearpointneuro.comir.clearpointneuro.com. (They singled out PTC Therapeutics’ AADC gene therapy as the first approved under such an expedited program, with others in the queue).
- The IGNITE consortium – a ClearPoint-sponsored working group of top neurosurgeons – published peer-reviewed guidelines on gene therapy delivery, reinforcing ClearPoint’s thought leadership in the fieldir.clearpointneuro.com.
- Perhaps most striking: in July 2025 alone, 17 patients worldwide were treated via ClearPoint-enabled neuro-infusions across 11 different drug trialsir.clearpointneuro.com. This was a record monthly volume of such cases and demonstrates the breadth of the company’s engagement (60+ active partners as noted). Each of those cases likely involved the sale of consumable cannulas and support services.
- To support future scaling, new ICD-10 procedural codes for neuro infusions took effect on October 1, 2025ir.clearpointneuro.com. This is a behind-the-scenes win that will help hospitals bill and track gene therapy delivery procedures, smoothing the path for eventual commercialization of more therapies (and, by extension, ClearPoint’s billing for its tools).
- Regulatory Clearances & Global Expansion: ClearPoint has been steadily expanding its regulatory footprint:
- In September 2025, the FDA granted clearance to expand the labeling of the ClearPoint Prism Laser System to 1.5 Tesla MRI scannersnasdaq.com. Previously, Prism was cleared only for 3.0T MRIs, which limited its use to certain advanced hospitals. Now that it’s cleared for the more common 1.5T machines, ClearPoint can market Prism to the majority of MRI-equipped hospitals (1.5T systems account for ~60% of clinical MRI use in the U.S.nasdaq.com). This significantly “increases ClearPoint Prism’s market opportunity” domesticallynasdaq.com. It’s a timely win as laser ablation for brain tumors and epilepsy gains traction as a minimally invasive alternative to open surgery.
- On October 6, 2025, ClearPoint announced its SmartFlow delivery products are now cleared in 34 countries worldwidestockanalysis.com. This likely includes much of North America, Europe (the SmartFlow cannula received EU CE Mark under MDR in early 2025), and parts of Asia/Pacific. The company noted that this broad regulatory acceptance “de-risk(s) BioPharma partners’ pathways towards achieving global scale and standardization”stockanalysis.com. In other words, if a partner’s drug works, ClearPoint’s device is already approved in key markets to deliver it. This is a selling point when courting new partners and may provide a competitive moat.
- ClearPoint also achieved EU MDR certification for its products in Feb 2025 (meeting Europe’s stricter regulations), and clearances for various software and hardware updates (e.g., ClearPoint Navigation software 3.0 got FDA clearance in Jan 2025ir.clearpointneuro.com). Each clearance incrementally improves or extends the platform (for instance, Software 3.0 and a new SmartFrame OR stereotactic device were rolled out to integrate ClearPoint into standard operating rooms without MRI, using intraoperative CT guidance) – all geared toward making ClearPoint’s platform more flexible and widely adoptable.
- New Robotic Neurosurgery System: On October 1, 2025, ClearPoint revealed it is prototyping a Robotic Neuro-Navigation Systemstocktitan.net. This system is designed to mount on a KUKA medical robotic arm and assist in automated, precision targeting during cranial procedures. It offers three modes: traditional MRI-guided manual operation, a new intraoperative CT (iCT) guidance mode called ClearPoint 3.0, and a future fully robotic-assisted mode – all integrated through the same ClearPoint software platformstocktitan.net. A prototype was demonstrated at the CNS (Congress of Neurological Surgeons) annual meeting in Los Angeles in mid-October 2025stocktitan.net. The move into robotics aims to improve consistency and throughput for high-volume therapy delivery. As more gene and cell therapies reach the market, hospitals may need to perform many similar brain infusions; robotic assistance could enable faster procedures with fewer errors. ClearPoint stated the goal is to provide “consistency and flexibility” for pharma partners preparing for “increased commercial case volumes”stocktitan.net. While this robotic system is still in development (not yet FDA-approved as of Oct 2025stocktitan.net), it represents a new product category and a potential future revenue stream (robotic hardware sales or leases). It also keeps ClearPoint competitive with firms like Renishaw and Brainlab, which offer robotic neurosurgical tools – ensuring ClearPoint can match any automation trends in neurosurgery.
- Leadership & Corporate Developments: As mentioned, Dr. Paul Larson (a neurosurgeon with deep stereotactic surgery experience) joined as CMO in late September 2025ir.clearpointneuro.com. His role is likely to guide clinical strategy and training as the company scales up in hospitals. On the corporate side, ClearPoint has opportunistically raised capital to fund growth. In May 2025 it entered a major financing deal with Oberland Capital, securing $30M in debt upfront plus access to $75M more over coming yearsir.clearpointneuro.com. Oberland also took an equity stake, buying shares at $12.69 (a premium at the time)ir.clearpointneuro.com. This infusion more than doubled ClearPoint’s cash (to $41.5M as of mid-2025, from $20M at 2024’s end)ir.clearpointneuro.comir.clearpointneuro.com. Management indicated the funds will fuel new product launches, a faster installed-base buildout, and support for partner trials scaling upir.clearpointneuro.com. This creative financing (a mix of debt, royalty, and equity) was seen as a strong endorsement: “ClearPoint…has a market leading portfolio…and a large and growing pipeline of…partnerships…we look forward to helping the Company achieve its long-term objectives,” said an Oberland partnerir.clearpointneuro.com.
- M&A or Partnerships: There have been no major acquisitions by ClearPoint in 2025 (the company’s growth has been organic and through collaborations). However, one notable partnership deal was announced in May 2025: a multi-year exclusive licensing agreement with UCSF (University of California, San Francisco) to co-develop a novel radial™ cell delivery device for the brainstocktitan.net. This hints at pipeline products beyond the current cannula – possibly a next-gen device to distribute cells more broadly in brain tissue. Additionally, ClearPoint congratulated partner Aspen Neuroscience in mid-2024 for using ClearPoint in all patients of its Parkinson’s cell therapy trialir.clearpointneuro.com, indicating deepening ties with cutting-edge biotech ventures.
In sum, late 2025 finds ClearPoint Neuro at an inflection point: its core technology is moving from experimental to commercial use, its product line is expanding (software updates, new robotics), and its global/regulatory footprint is largely in place. The recent news flow underscores both the opportunities (multiple shots on goal with partner therapies, higher procedure volumes, new markets) and the challenges (managing rapid growth, maintaining a strong balance sheet, and navigating any partner setbacks).
Financial Analysis: Revenue, Earnings and Balance Sheet
ClearPoint Neuro’s financial profile reflects an emerging growth company investing for future payoff. While revenue is climbing at an impressive rate, the company is not yet profitable and incurs significant operating losses as it builds out its platform.
Revenue Growth: ClearPoint has delivered consistent top-line growth for over a decade. In 2024, revenue was $31.4 million, up 31% from 2023nasdaq.com – marking the company’s 10th consecutive year of revenue growth. For full-year 2025, management forecast $36–$41 million in revenuenasdaq.com, which would be ~15%–30% growth on 2024. Through the first half of 2025, ClearPoint was on track: H1 revenue totaled about $17.1M (Q1 $7.9M, Q2 $9.2M)accessnewswire.com. Q2 2025’s $9.2M was a record quarter and up 17% year-over-yearaccessnewswire.com. This growth is fueled by:
- Increased device sales to hospitals (as installed base grows, each new site contributes capital sales and recurring disposables revenue).
- Rising procedure volumes using ClearPoint products – more DBS, laser, biopsy cases, and especially more clinical trial cases in the biologics segment.
- Service and partnership revenue – e.g., pharma partners paying for ClearPoint’s support in trials, development services, etc.
ClearPoint also sometimes earns one-time or milestone fees (for instance, licensing or development agreements). However, the bulk of revenue is from product sales (capital and disposables). In Q2 2025, ClearPoint noted neurosurgical navigation & therapy product revenue grew 33% to $3.4Maccessnewswire.comaccessnewswire.com, while the balance of revenue came from biologics/drug delivery related sales and services. The mix of revenue is shifting increasingly toward the biologics segment as more partners enter trials.
Earnings and Margins: The company remains in the red as of 2025. In 2024, ClearPoint’s net loss was about $18.9 millionstockanalysis.com, slightly less negative than 2023’s loss. For 2025, analysts forecast around –$1.00 EPS (full-year) which would equate to roughly a $28–$30M net loss, given ~28M shares outstandingmarketbeat.com. The losses are driven by heavy operating expenses:
- R&D: ClearPoint is investing in new products (e.g. robotics, software, extended compatibility) and supporting 60+ partner projects (which requires an R&D and clinical support team). This is crucial spending to stay ahead technologically, but it weighs on current earnings.
- Sales & Marketing: As the company expands globally and into more hospitals, it needs a larger salesforce and clinical support staff. Training neurosurgeons and supporting initial cases at each new site is resource-intensive.
- G&A: Growing a public company (with regulatory, quality, and administrative overhead) adds costs too.
Gross margins on product sales are healthy (likely in the 70-80% range for disposables, somewhat lower for capital equipment), but the volume is not yet high enough to cover operating expenses. The forward P/E is negative (not meaningful) and standard profitability metrics look weak – e.g., return on equity was –85% in recent datadirectorstalkinterviews.com. However, this is expected for a high-growth medtech. ClearPoint’s situation is reminiscent of other emerging device companies that operated at a loss until their technology achieved broad adoption.
One bright spot: the company’s gross margin and operating leverage should improve if/when partner therapies move from clinical trial phase to commercial launch. At that point, ClearPoint would sell larger quantities of cannulas and likely command service contracts for training dozens of new sites, all of which could quickly multiply revenue without a proportional increase in overhead. Investors bullish on CLPT are essentially betting that current losses are “investment mode” spending that will pay off in the form of high-margin consumables revenue later.
Cash & Balance Sheet: ClearPoint had $41.5 million in cash and equivalents as of June 30, 2025, up from $20.1M at the end of 2024stockinvest.us. This boost came from the May 2025 Oberland Capital deal, which immediately provided $30M debt and $3.5M equity fundingir.clearpointneuro.comir.clearpointneuro.com. The Oberland notes are a form of debt financing that will need to be repaid (likely with interest and possibly a royalty component), so they add to liabilities. Indeed, ClearPoint’s debt-to-equity ratio jumped to 1.46 after this financingmarketbeat.com, indicating a significant leverage increase. However, the terms appear flexible (additional tranches only if needed) and the cash infusion was considered necessary to support growth. The company also raised $6.5M from a public equity offering in early 2025 at $6.50/share (a relatively small dilution given the stock’s subsequent rise)ir.clearpointneuro.com.
With ongoing losses, ClearPoint’s cash burn is something to monitor. Free cash flow was about –$2.8M in a recent quarterdirectorstalkinterviews.com. If we annualize that, the current cash could fund roughly 2-3 years of operations. The Oberland facility’s additional $75M available provides a cushion if new capital is needed for expansion or if losses continue longer than expected. Still, investors should expect the company to eventually either reach breakeven (if revenue ramps up as therapies commercialize) or potentially tap equity markets again in a few years, especially if new growth projects arise.
Key Financial Ratios: ClearPoint’s stock valuation reflects future expectations:
- Price/Sales (P/S) ratio is elevated (roughly 15x 2025 sales at recent prices), high even for medtech – showing the market is pricing in significant growth beyond current revenues.
- The P/E is not applicable due to negative earnings. On a Price/Cash Flow or EV/Revenue basis, CLPT also looks pricey compared to established device firms. But such comparisons are tricky given the company’s unique position and growth stage.
- Liquidity ratios are strong: Quick ratio ~6.45 and current ratio ~7.3marketbeat.com, meaning ClearPoint has ample short-term liquidity (mostly cash) relative to its short-term liabilities. This is a positive sign that they can fund near-term operations and commitments easily.
- Profitability ratios are negative (e.g., net margin –66%marketbeat.com), underscoring the current focus on growth > profits.
In summary, ClearPoint’s financial story is top-line growth and strategic investment. Revenues are rising steadily and are expected to accelerate if partner programs succeed, but in the meantime the company is spending aggressively, resulting in ongoing losses. The balance sheet has been fortified by recent financings, though debt is higher. Analysts generally seem comfortable with this profile at present, as long as ClearPoint continues executing on growth milestones. The trajectory toward eventual profitability likely hinges on expanding the installed base and seeing some partner therapies progress to commercialization (driving a jump in disposable sales). Until then, quarterly results will show a mix of solid revenue gains and red ink on the bottom line – something long-term investors in the sector are accustomed to.
Products, Pipeline and Technology Outlook
ClearPoint Neuro’s value proposition lies in its innovative product portfolio and robust pipeline of new technologies, all aimed at revolutionizing neurosurgical therapy delivery. Here we breakdown the key products and what’s coming next:
- ClearPoint Neuro Navigation System (Integrated MRI-Guided Surgery Platform): This is the company’s foundational product, essentially a “GPS for the brain” used inside MRI suites. It consists of software, MR-compatible hardware (like the SmartFrame stereotactic head frame), and instrumentation. The system allows real-time visualization and guidance for placing instruments in the brain. It is FDA-cleared and CE-marked and has been used in thousands of procedures to dateir.clearpointneuro.com. Key applications include:
- Deep Brain Stimulation (DBS) lead placement: ClearPoint enables neurosurgeons to precisely implant electrodes for Parkinson’s, tremor, epilepsy etc., under MRI guidance instead of traditional techniques. This can improve accuracy and patient comfort (no need for awake surgery)cstproxy.comcstproxy.com.
- Laser Interstitial Thermal Therapy (LITT): ClearPoint’s platform guides the insertion of laser fibers into tumors or epileptic lesions and monitors ablation in real time via MRI thermography. ClearPoint’s Prism Laser Therapy System, launched 2022, works in conjunction with this navigation platform. After its latest FDA clearance, Prism can be used with most hospital MRI scanners (1.5T or 3T)nasdaq.com, putting it in direct competition with Medtronic’s Visualase and Monteris’ NeuroBlate systems for minimally invasive neurosurgery.
- Brain Biopsy and Aspiration: For diagnostic biopsies of deep brain lesions, the system aids trajectory planning and needle placement, increasing safety and yieldcstproxy.com.
- Drug Delivery: The navigation software and hardware guide the placement of the SmartFlow cannula (see below) for targeted infusion of therapeutics. The software provides planning for drug distribution, avoidance of critical structures, and monitoring of infusate via MRI.
- SmartFlow® Cannula (Therapy Delivery Injection Device): This is a patented, MRI-compatible needle/catheter system that can precisely infuse drugs or gene therapy vectors into specific brain targets. It has a step-designed tip to prevent reflux of the infused agent. SmartFlow is central to ClearPoint’s biologics partnerships. In 2024, the SmartFlow cannula received an FDA De Novo clearance as a combination product for delivering PTC’s AADC gene therapy into the putamenir.clearpointneuro.com, a landmark regulatory approval that essentially validated the device for CNS drug delivery. SmartFlow is now being used in clinical trials for many other therapeutics – for example, gene therapies for Huntington’s disease (AMT-130 by uniQure), Parkinson’s (Voyager and others), glioblastoma (multiple immunotherapy trials), etc. It’s also the device used in the first commercial gene therapy case (KEBILIDI) as noted earlier. Because it’s the only FDA-cleared cannula for gene delivery to the brain so far, SmartFlow has a head start in this niche. Internationally, SmartFlow and related ClearPoint delivery tools have garnered approvals in dozens of countriesstockanalysis.com, meaning partners can use the same device in trials worldwide. This global presence is a strategic asset – drug developers prefer not to validate a new device from scratch in each region, so ClearPoint’s established regulatory approvals make it an attractive choice. In the pipeline, ClearPoint and UCSF’s collaboration on a “radial” infusion device suggests a next-gen cannula that might distribute therapeutic cells or fluids in a radial fashion (perhaps to cover larger areas or multiple sites from one insertion). This could be important for diseases that affect broader brain regions, like Parkinson’s (where you may want to cover multiple sites in the putamen). No timeline is given, but it shows the company is innovating beyond the current straight-line cannula design.
- ClearPoint Prism® Neuro Laser System: A laser ablation therapy platform for destroying unwanted tissue in the brain (tumors, seizure foci) with heat. Prism is based on tech licensed from CLS (Sweden) but marketed by ClearPoint in the U.S. Since its clearance in late 2022, ClearPoint has been commercializing Prism as part of its neurosurgery offering. The distinguishing feature is full MRI guidance and the potential for integration with ClearPoint navigation. The recent FDA clearance for 1.5T MRI compatibilitynasdaq.com removes a barrier to adoption, so we may see acceleration in Prism system installs. ClearPoint’s Q2 2025 results indicated that neurosurgery product revenues (which include laser systems) were climbing 33% YoYaccessnewswire.comaccessnewswire.com, possibly reflecting initial Prism sales. Prism competes in a space dominated by Medtronic’s Visualase and Monteris Medical’s NeuroBlate. ClearPoint will leverage its existing hospital relationships and MRI integration to carve out share. Additionally, ClearPoint uniquely can offer laser ablation plus drug delivery on the same platform, which competitors do not – a hospital that buys ClearPoint for laser gets the capability for drug trials, and vice versa.
- Robotic Neuro-Navigation (in development): Announced in October 2025, ClearPoint’s robotic system will use the KUKA LBR Med robotic arm to execute surgical trajectories under the guidance of ClearPoint softwarestocktitan.net. This could automate the positioning of instruments like the SmartFlow cannula or laser probes, reducing manual variability. The prototype supports “robotic-assisted technique” as a third modality (after manual MRI and iCT guidance)stocktitan.net. Robotic assistance could shorten procedure times and improve accuracy – important as volumes grow. Competitors such as Renishaw’s Neuroinspire/Neuromate robot and Brainlab’s robotic systems are already in some ORs, so ClearPoint is ensuring it won’t be left behind. The fact that they demonstrated a prototype at a major neurosurgery meeting in 2025 suggests a tangible progress, but full commercialization may be a couple of years out (pending further development and regulatory clearance). If successful, this product will broaden ClearPoint’s revenue streams (likely capital sales or leasing of the robotic unit, on top of disposables).
- Software Ecosystem & Data: ClearPoint’s software platform is a less heralded but crucial part of its offering. It integrates planning (using patient MRI scans to plan trajectories and infusion volumes), intra-procedure imaging, and even post-procedure analytics. The company has hinted at incorporating AI and digital tools (e.g., AI-informed quality control documentation as mentioned by the CEOir.clearpointneuro.com) to ensure consistent results. Over time, the data collected from many procedures could feed back into improved targeting and outcomes (a potential differentiator vs. competitors). ClearPoint also engages in preclinical services – they have an R&D lab that works with pharma companies to optimize infusion protocols in animal models and early studiesir.clearpointneuro.com. This positions them not just as a device vendor, but as a partner in therapy development.
Looking ahead, ClearPoint’s pipeline aligns tightly with the needs of the burgeoning neuro-therapeutics field:
- More advanced delivery devices (like multi-port cannulas, radial diffusers, etc.) to accommodate different therapy types (genes, stem cells, oncolytic viruses, etc.).
- Ancillary products such as navigation aids for the spine (they mention brain and spine navigationir.clearpointneuro.com; perhaps extensions to spinal cord delivery in the future).
- Continued software enhancements for planning complex deliveries (imagine multi-target infusions for disorders like Parkinson’s that affect circuits across the brain).
- Training and support programs (like IGNITE consortium) that ensure the user base (surgeons) are up to speed and comfortable adopting these novel procedures – indirectly expanding product adoption.
In conclusion, ClearPoint’s technology suite is increasingly comprehensive: from planning software to drilling devices, infusion cannulas, laser probes, and soon robots – all under one unified platform. This “one-stop-shop” approach for MRI-guided neuro interventions gives ClearPoint a competitive edge in becoming the standard operating system for brain interventions. As more therapies advance, the company’s product roadmap appears well-matched to support them, which is a primary reason analysts and investors are excited about its future potential.
Analyst & Expert Commentary: What the Street is Saying
ClearPoint Neuro’s recent success has caught the attention of financial analysts and industry experts, who largely have a positive outlook on the company. Here we compile some commentary and perspectives:
Wall Street Ratings and Targets: The stock is currently universally rated “Buy” by the few analysts formally covering itdirectorstalkinterviews.com. MarketBeat reports at least 4 analysts with an average 12-month price target of $19.67–$29.00 (estimates vary)marketbeat.com, but those targets appear outdated given the stock’s rapid rise. More recent analyses peg the consensus target around $28–$30, essentially in line with where the stock traded in late Octoberdirectorstalkinterviews.com. For example, DirectorsTalk Interviews noted “analysts have set a target price range of $28.00 to $30.00, providing an average target of $29.00”directorstalkinterviews.com. Similarly, StockAnalysis.com lists a $27.67 target (74% above a prior price when CLPT was lower)stockanalysis.comstockanalysis.com.
This bullish stance is underpinned by the company’s strong growth and unique market position. Analysts highlight that ClearPoint is pioneering a new category. A MarketScreener consensus comment summarizes that sentiment with a “BUY” consensus and ~+25% upside impliedintellectia.ai.
Bullish Arguments: Proponents of the stock often describe ClearPoint as akin to “selling picks and shovels in a gold rush.” The gold rush here is the wave of gene and cell therapies for neurological diseases. ClearPoint doesn’t have to develop the therapies (avoiding binary drug trial risk), but it profits from supplying the tools that all those therapy developers need. This model can scale broadly if multiple drugs succeed.
A Seeking Alpha contributor in October 2025 titled “ClearPoint Neuro: AMT-130 Highlights A Bright Future” noted that “ClearPoint’s stock surged after uniQure announced strong Huntington’s data, highlighting the value of its drug delivery platform”stockanalysis.comstockanalysis.com. The article emphasized that the positive Huntington’s trial results effectively validated ClearPoint’s technology, since the trial used the SmartFlow device to deliver the gene therapy. With that validation, ClearPoint is “positioned” as a key player for many such therapies coming down the pipelinestockanalysis.com. Although uniQure’s program hit an FDA snag later (more on that in Risks), the initial data underscored the potential demand for ClearPoint’s platform if these treatments work.
Another Seeking Alpha piece called “Selling The Tools For Brain Therapies” (September 2025) pointed out that ClearPoint’s share price had “surged over 100%” following the Huntington’s data and framed the company’s opportunity succinctly: “CLPT’s SmartFlow cannula is already validated by [the] AMT-130 data… [ClearPoint is] selling the tools for brain therapies”stockanalysis.comstockanalysis.com. The author’s thesis was that rather than betting on which biotech will win, invest in the company that supplies all of them. This mirrors historical cases in other sectors (e.g., Illumina supplying gene sequencers to genomics researchers).
Financial commentators also appreciate ClearPoint’s expanding recurring revenue. Once ClearPoint’s systems are installed in a hospital, that site generates ongoing revenue from disposables and service, and if new therapies emerge, volumes per site can increase. As one analysis noted, revenue growth of ~17–20% (as seen in 2025) coupled with high gross margins makes for an attractive long-term model, provided the company can eventually trim its lossesdirectorstalkinterviews.comdirectorstalkinterviews.com.
DirectorsTalk’s October 30 report praised ClearPoint’s strategic partnerships and collaborations with “notable institutions like Philips, UCB, Johns Hopkins, and UCSF” as supporting its growthdirectorstalkinterviews.com. These lend credibility and suggest multiple avenues of expansion (e.g., Philips could help distribute ClearPoint’s tech alongside MRI machines; UCSF fosters innovation). The article noted that while financial metrics like EPS are negative, the revenue growth and analyst bullishness present a compelling storydirectorstalkinterviews.comdirectorstalkinterviews.com.
Cautious Notes: Despite the overwhelmingly positive tone, experts do flag some concerns. One risk repeatedly mentioned is execution – i.e., ClearPoint must continue placing systems and growing utilization to justify its valuation. A Seeking Alpha analysis of Q2 2025 warned “there is a risk that ClearPoint fails to place its target number of systems in 2025,” which could cause the stock to underperformseekingalpha.com. Essentially, if hospital adoption slows or any reason (budget constraints, competition), revenue growth could disappoint.
Another point often raised is competition and innovation risk. As M.V. Cunha (a biotech investor) noted, “Competitors like Medtronic, Brainlab, or Renishaw could invest more heavily in neurosurgical delivery or develop MRI-compatible tools”, which would challenge ClearPoint’s first-mover advantagemvcinvesting.substack.com. So far, none of those giants have a directly competing comprehensive platform, but the barrier to entry isn’t insurmountable for them if the market is proven large. This is on analysts’ radar for the long term – ClearPoint needs to move fast to entrench itself (which it is attempting via advanced products and partnership integrations).
Financially, the lack of profitability is a common caveat in reports. Simply Wall St, in an October 2025 commentary titled “Is CLPT a Risky Investment?”, pointed out the company’s negative earnings and ROE (return on equity – ClearPoint’s ROE was around –86% last yeardirectorstalkinterviews.com) as indicators that it’s still far from turning a profit. However, they also noted revenue growth of 20%+ as a mitigating factor. The overall sentiment: the company has “yet to overcome [the] hurdle” of profitabilitydirectorstalkinterviews.com, but as long as it can finance its growth and expand the top line, investors appear willing to be patient.
Insider activity is another area experts scrutinize. MarketBeat highlighted that CEO Joe Burnett sold 26,463 shares in October 2025 at ~$26.98, trimming about 10% of his stakemarketbeat.commarketbeat.com. Such insider sales can be interpreted in many ways; MarketBeat noted it “indicat[es] potential internal shifts in confidence”marketbeat.com. It’s not uncommon for founders/executives to take some profit after a big run-up, but investors will watch if insiders continue selling or if they hold onto the majority of their shares (a sign they believe more upside is ahead).
Lastly, macro considerations: interest rate conditions and risk appetite in 2025 have affected high-growth stocks. If market conditions turn unfavorable for loss-making tech/healthcare firms, ClearPoint’s stock could see compression. Some analysts mention that volatility is high and that ClearPoint’s valuation leaves little room for error – any slowdown in growth or adverse trial news could cause a sharp pullback, as evidenced by the late-October dip and post-Nov 3 reaction to uniQure’s FDA news.
Overall, though, the expert commentary can be summed up as “cautiously optimistic.” The bulls believe ClearPoint is building something special and could become an indispensable player in a burgeoning field, while the cautious voices remind us that the company’s current success is largely prospective – tied to therapies yet to be approved and volumes yet to materialize. The next 1-2 years of execution will be crucial to validate the bullish thesis.
To quote DirectorsTalk’s conclusion: “ClearPoint’s commitment to advancing brain surgery technology, coupled with its promising growth metrics and analyst endorsements, makes it a stock worth watching… As with any investment, due diligence and careful consideration of the associated risks and opportunities are essential.”directorstalkinterviews.com.
Strategic Outlook and Market Positioning
ClearPoint Neuro’s strategic outlook is built on the idea that it can be the central technology provider for a new era of neurosurgical therapies. If we zoom out, the company’s strategy is to position itself as:
- The standard platform in operating rooms for MRI-guided interventions, and
- The go-to partner for pharma/biotech for CNS drug delivery.
Achieving these aims would give ClearPoint a strong, defensible niche with high switching costs (once hospitals and drug makers commit to ClearPoint’s system, they are likely to stay with it, provided it continues to meet their needs).
Competitive Landscape: ClearPoint operates at the crossroads of medical devices and biotech, so it faces different sets of competitors in each realm:
- In neurosurgical navigation and therapy devices, major medtech companies are potential rivals. Medtronic, Boston Scientific, and Abbott dominate deep brain stimulators (DBS implants) but they do not supply the MRI navigation – indeed, ClearPoint’s system can complement those companies’ DBS hardware by improving placement. For laser ablation, as discussed, Medtronic (Visualase) and Monteris (NeuroBlate) are incumbents; ClearPoint is the newer entrant but touts MRI integration advantages. Brainlab (a German surgical tech firm) offers navigation systems primarily for CT-based neurosurgery and has some laser guidance solutions; Renishaw (UK) has a neurosurgery robot (Neuromate) and has developed its own drug delivery cannula (Neuroinfuse) used in some trials. ClearPoint specifically acknowledges Brainlab and Renishaw as companies with “navigational platforms and cannulas” for drug delivery under MRIcstproxy.com, highlighting them as direct competitors in the biologics space. However, neither has the combined hardware/software suite that ClearPoint does, nor the extensive clinical usage to date. In essence, ClearPoint currently holds a first-mover advantage in integrated MRI-guided drug delivery – a niche that the big generalist medtech players haven’t fully addressed yet.
That said, the risk is not lost on ClearPoint: a deep-pocketed competitor could decide to develop a similar MRI-based platform or even acquire one of the smaller players. ClearPoint’s defense is to move fast and entrench its tech with as many pharma programs and hospital sites as possible, creating a de facto standard. By actively partnering with 60+ therapy developers and engaging neurosurgical KOLs (through IGNITE, etc.), they are trying to make their ecosystem the preferred choice before competitors mobilize.
Market Position vs. Competition: ClearPoint’s positioning can be summarized as “highly specialized and innovative, but smaller scale” relative to large medtech. Many competitors have far greater resources, but are spread across many product lines, whereas ClearPoint focuses narrowly on neuro intervention. The company leverages partnerships (with Philips for MRI compatibility, with CLS for laser tech, with academic centers for R&D) to punch above its weight. One advantage ClearPoint has is that its technology is patent-protected and FDA-cleared in areas where others might still be in development. For example, Renishaw’s Neuroinfuse drug delivery system, while used in some EU trials, did not have broad FDA clearance as of 2025, whereas ClearPoint’s did for at least one indication (AADC gene therapy) and is in routine trial use. This regulatory lead can be important.
Additionally, ClearPoint’s multi-faceted approach – hardware, software, support – means a competitor has to match them on several fronts. A hospital considering MRI-guided neurosurgery might see ClearPoint as a one-stop solution. Even if a competitor sells a robot or a navigation software alone, they may not have the integrated package.
Strategic Partnerships: Another pillar of ClearPoint’s strategy is alliances:
- Partnering with pharma companies ensures that if a therapy succeeds, ClearPoint rides along to commercial adoption. Each partner also validates ClearPoint’s tech to some degree, and there’s a network effect (more partners = more trial data = more proof that ClearPoint works and is safe).
- Partnering with imaging companies (like Philips, Siemens potentially) could help embed ClearPoint’s software into MRI systems or sales channels. ClearPoint’s mention of Philips suggests some collaboration, possibly to ensure compatibility or co-marketing.
- Working with leading hospitals and neurosurgeons (e.g., Cleveland Clinic, Boston Children’s, etc. which have been early adopters) builds credibility and creates centers of excellence that train others.
- Notably, the financing partnership with Oberland Capital is strategic financially – it gives ClearPoint growth capital without immediate equity dilution, and Oberland’s structure (which often involves royalties) aligns them to ClearPoint’s success in generating sales from approved therapies.
Growth Strategy:
- Near-term (1–2 years): ClearPoint will focus on expanding its installed base in neurosurgery centers. The more sites have ClearPoint systems, the easier it is for pharma partners to plan trials (knowing hospitals have the capability). The company likely has internal targets for system placements each quarter. They’ll also push adoption of the Prism laser in current and new sites as an additional revenue generator. Simultaneously, supporting partner trials to successful outcomes is key – any high-profile trial result that leads to FDA approval (akin to the AADC gene therapy) will boost ClearPoint’s credibility and likely drive more orders (hospitals will prepare to treat patients, and new biotechs will sign on seeing the success).
- Mid-term (3–5 years): We could see the first wave of partner therapies hitting the market (by 2026-27, candidates like Voyager’s Parkinson’s gene therapy, or others in Phase 2 now, might reach approval if all goes well). ClearPoint then transitions from mostly trial revenue to also commercial therapy support – meaning potentially hundreds of patients per year getting treatments via ClearPoint cannulas, each generating thousands in disposable sales. The company’s revenue could inflect upward significantly at that point. In preparation, ClearPoint will need to scale manufacturing of disposables and training programs for many new sites (hence the interest in robotics to help handle volume). International expansion will also kick in fully if therapies launch globally – ClearPoint may establish more direct sales or distributor networks in Europe, Asia etc., building on the regulatory approvals in 34 countries.
- Long-term: ClearPoint envisions itself as a platform tech in every major neurosurgery department. If neuro-interventions continue to advance (think gene therapy for Alzheimer’s one day, or widespread use of regenerative cell therapies for stroke, etc.), ClearPoint wants its systems to be as common as, say, angiography suites for cardiology. Achieving this will require staying ahead on innovation and possibly broadening applications (e.g., maybe enabling spinal cord injections for ALS, or minimally invasive approaches for other CNS disorders). The total addressable market could expand from tens of thousands of procedures to hundreds of thousands if multiple common conditions adopt interventional therapies.
Strengths and Weaknesses: Strategically, ClearPoint’s strengths are its pioneering technology, growing body of clinical evidence, and alignment with a major trend (personalized CNS medicine). It also benefits from relatively little direct competition in its specific niche at present. Its weaknesses include its small size (limited sales force, limited ability to self-fund indefinitely), reliance on external success (if partners’ drugs fail, ClearPoint doesn’t directly generate outcomes to drive demand), and exposure to any slowdown in innovation in neuro therapeutics. If, hypothetically, CNS gene therapies hit a roadblock or safety issue industry-wide, ClearPoint’s growth could stall.
Market Position vs. Pharma approach: One interesting strategic angle – some pharmaceutical companies might try to develop their own delivery methods to reduce dependency on a single vendor like ClearPoint. For example, they could work with academic labs to design custom catheters for a specific trial. However, given regulatory hurdles and the costs, most have opted to use ClearPoint’s proven device. ClearPoint being neutral and widely used actually makes life easier for regulators too (they gain familiarity with one system). So ClearPoint’s broad adoption so far actually discourages one-off approaches. It’s somewhat analogous to how almost all CAR-T cell therapies use the same kind of leukapheresis machines and infusion processes – standardization is preferred.
In conclusion, ClearPoint’s strategic plan is to embed itself deeply in the emerging neurotherapeutics ecosystem so that it becomes indispensable. The company is fairly on track in executing this plan: it continues to notch technical clearances, partner wins, and first-of-kind achievements. The next few years will test whether it can maintain its lead as competitors eye the space. If successful, ClearPoint could end up being the equivalent of an “Illumina of neuro-intervention” – a picks-and-shovels leader with a dominant market share in a growing field. If not, it could still be an attractive acquisition target for a larger medtech or pharma looking to instantly gain expertise in MRI-guided therapy delivery.
Risks and Challenges
No investment is without risks, and ClearPoint Neuro – despite its promising story – faces several key risks and challenges that investors should keep in mind:
1. Clinical Trial and Partner Dependency: Perhaps the most prominent risk is that ClearPoint’s success is tied to the success of its partners’ drug programs. While diversification across 60+ programs reduces single-trial risk, a broad industry setback (e.g., unforeseen safety issues with gene therapy to the brain) could dampen demand for ClearPoint’s technology. The events of November 3, 2025 illustrate this risk starkly: The FDA gave negative feedback on uniQure’s Huntington’s gene therapy (AMT-130), despite earlier positive data. This “drastic change” in regulatory stancekfgo.com not only sent uniQure’s stock crashing ~68%kfgo.com, but also raised concern about the viability of that approach in the near term. ClearPoint’s cannulas were used in AMT-130’s trials, so a major delay or cancellation there means one less near-term commercial opportunity. More broadly, it reminds investors that ClearPoint doesn’t control the end-market timelines – approvals can be delayed or denied for reasons unrelated to ClearPoint’s device (efficacy, safety of the therapy, etc.). Mitigant: ClearPoint’s broad portfolio of partners provides multiple “shots on goal.” For instance, even if uniQure’s program is delayed, others (like PTC’s AADC gene therapy or upcoming trials in Parkinson’s or epilepsy) can carry the torch. Nonetheless, a general cooling in CNS gene therapy enthusiasm or funding could slow ClearPoint’s growth materially.
2. Adoption Risk & Market Development: ClearPoint is effectively creating a new market – MRI-guided interventions were not routine in many hospitals before. There’s a risk that some hospitals or surgeons are slow to adopt these new techniques. For example, neurosurgeons comfortable with traditional frame-based systems or other guidance might be hesitant to switch to ClearPoint, especially given the need for MRI suite time and new training. ClearPoint has to continually educate and prove out the advantages (accuracy, patient comfort, new capabilities like drug delivery). If the company fails to place its targeted number of new systems (as one analyst cautionedseekingalpha.com), revenue growth could stall. Relatedly, if the expected wave of commercial therapies takes longer than hoped, hospitals might not feel pressure to invest in ClearPoint systems immediately. Essentially, timing mismatches could occur – ClearPoint might build capacity anticipating therapies that then get delayed. This could lead to under-utilization of systems and weaker disposable sales, affecting profitability.
3. Competition and Technological Risk: While ClearPoint currently enjoys a lead, the competitive moat may not be wide if larger players decide to enter. Giants like Medtronic or Brainlab have resources to develop similar products. Medtronic, for instance, could integrate drug infusion capabilities into their existing neurosurgery platforms or leverage their installed base to push a competing product if they see a big opportunity. Also, academic groups (often funded by government or foundations) have explored alternative drug delivery methods (like convection-enhanced delivery catheters) – one could be commercialized and bypass ClearPoint’s tech. ClearPoint’s patent portfolio and first-mover status provide some protection, but not indefinitely. There’s also technology obsolescence risk: MRI-guided delivery is one approach, but what if a breakthrough enables non-surgical delivery of these therapies (for example, focused ultrasound to open the blood-brain barrier temporarily)? Such a development could reduce the need for direct injections. ClearPoint must continue innovating (as it is with robotics and new cannulas) to stay ahead, but innovation is costly and not guaranteed to succeed.
4. Financial Risk and Dilution: ClearPoint’s operations are not yet self-funding. It relies on external capital to cover cash burn. While recent financings have bolstered the balance sheet, heavy ongoing investments might eventually require further capital raising. The Oberland Capital deal, for instance, includes debt and royalty components – future repayment or royalty outflows could weigh on financials. If ClearPoint needs to draw the additional $25M or $50M tranches, that increases debt. And if they needed more equity, issuing shares when the stock is volatile could dilute existing shareholders. Moreover, carrying a high debt-to-equity ratio (around 1.46)marketbeat.com is unusual for a small medtech and introduces risk if interest rates rise or covenants become an issue (though specifics of the note aren’t public, structured financings sometimes have conditions). In short, until profitability is reached or very clear, financing risk remains: the company might need more cash than expected if, for example, revenue ramps slower or if it undertakes a big new project.
5. Execution and Scaling Challenges: Transitioning from a small R&D-focused company to a commercial enterprise is non-trivial. ClearPoint’s headcount is relatively small (~115 peoplestockanalysis.com), which might be stretched thin to support dozens of trial sites globally, simultaneously launching new products (like Prism or the robot) and servicing installed devices. As demand grows, can they scale manufacturing of disposables reliably? Any quality control issues could be serious (imagine a faulty cannula impacting a patient treatment – that could harm reputation significantly). Also, the need to provide consistent training and support for every new center means ClearPoint must perhaps grow its clinical support staff and maintain high service levels. Customer satisfaction is key – neurosurgeons and biotech clients will only continue using ClearPoint if the product and support are excellent. A few bad experiences could slow momentum. Thus far, feedback seems positive, but the more they scale, the greater the operational complexity.
6. Regulatory and Legal Risks: Operating in the medical field means regulatory oversight at every turn. ClearPoint not only needs FDA clearances for its devices (which it has obtained and must maintain with quality compliance), but if it’s considered a part of combination products (as with the SmartFlow for AADC therapy), any regulatory issues with the device could impact the drug approval or vice versa. Also, if adverse events occur in trials or treatments (even if due to the drug), ClearPoint’s device might draw scrutiny. On the legal side, being in healthcare means potential liability – although likely covered by insurance, a patient injury claim related to device use could have financial or reputational repercussions. Intellectual property is another area: ClearPoint needs to defend its patents; conversely, it needs to ensure it’s not infringing others’. For instance, there have been various patents on infusion catheters – any dispute could impose costs.
7. Market Sentiment and Stock Volatility: This is more of an investor risk than a business risk, but worth noting: CLPT stock has been extremely volatile. Rapid swings can be emotionally challenging for investors and could lead to pressured stock prices independent of fundamentals (for example, a broad small-cap selloff could hit CLPT hard, or vice versa euphoria can swing it up). The company’s beta ~1.2 indicates more volatility than the marketmarketbeat.com. If one is considering CLPT as an investment, they must be prepared for significant ups and downs. There’s also a risk that if the stock price remains elevated but the company doesn’t yet have the earnings to back it up, it could be vulnerable to a sharp correction.
8. Acquisition Risk: Interestingly, one could posit a “risk” that ClearPoint becomes a takeover target. This could actually reward shareholders if at a premium, but it also might mean the company’s independent growth story gets cut short. A larger company acquiring it might slow down certain innovative aspects or integrate it in a way that’s less exciting for those banking on the high-growth standalone scenario. While not a risk in the traditional sense (unless an acquisition happens at a low premium due to some weakness), it’s something to be aware of in the medtech space where big fish often gobble up the smaller ones when they prove their model.
In weighing these risks, it’s clear ClearPoint is not a guaranteed success – it faces external dependencies and must execute nearly flawlessly to justify its stock surge. The company’s management appears aware of these challenges; for example, CFO D’Alessandro specifically noted the need to accelerate product introductions and hospital preparedness in line with partner demandsir.clearpointneuro.com, showing they know execution speed is vital. Likewise, the heavy focus on quality (new ICD-10 codes, published clinical guidelines) indicates they are proactively smoothing regulatory and adoption hurdles.
Investors and observers will be watching how ClearPoint navigates these headwinds. Many of the risks are typical for a high-growth medtech/biotech-adjacent firm. The upside potential – enabling revolutionary therapies and capturing a new market – has to be balanced against the possibility of scientific or commercial setbacks. At this juncture (late 2025), the risk-reward profile of CLPT remains high: significant reward if the company’s vision materializes, and significant risk if things go awry or simply take much longer than expected.
Sources:
- Nasdaq/RTT News – “ClearPoint Neuro Surges Nearly 40% – Retest 52-Week High?” (Company overview, FDA clearance, revenue guidance)nasdaq.comnasdaq.com
- MarketBeat – “CLPT Projected to Post Earnings on Nov 6, 2025” (Q3 expectations, stock move, insider sale, financial metrics)marketbeat.commarketbeat.com
- ClearPoint Neuro Press Release, Aug 4, 2025 – “Progression of Key Partner Milestones” (First commercial gene therapy infusion, 60+ partners, IGNITE surgeons’ quotes)ir.clearpointneuro.comir.clearpointneuro.com
- ClearPoint Neuro Press Release, Oct 6-7, 2025 – “Expanded International Clearances…34 Countries” and “Brain Tumor Laser Therapy Study Results” (Global regulatory reach, new therapy applications)stockanalysis.comstockanalysis.com
- StockAnalysis News – (Seeking Alpha summary) “ClearPoint: AMT-130 Highlights a Bright Future” (uniQure data impact, drug delivery validation)stockanalysis.com
- DirectorsTalk Interviews, Oct 30, 2025 – “CLPT: 20% Upside Potential” (Analyst targets, financial ratios, partnerships)directorstalkinterviews.comdirectorstalkinterviews.com
- Reuters (via KFGO), Nov 3, 2025 – “uniQure shares plummet as FDA says inadequate data for Huntington’s therapy” (FDA feedback, uniQure stock drop, prior surge)kfgo.comkfgo.com
- ClearPoint Neuro Investor Presentation/IR – (Oberland Capital financing details, CFO and partner quotes)ir.clearpointneuro.comir.clearpointneuro.com.